[Event Report] Invitation-Only Policy Dialogue Event “Considering Comprehensive Genomic Profiling from the Perspective of Patient Access: Utilizing the Medical Service Fee Reimbursement System and the Mixed Medical Services Program to Meet the Needs of Today” (July 14, 2025)
date : 7/29/2025
Tags: Cancer, NCDs, Precision Cancer Medicine
![[Event Report] Invitation-Only Policy Dialogue Event “Considering Comprehensive Genomic Profiling from the Perspective of Patient Access: Utilizing the Medical Service Fee Reimbursement System and the Mixed Medical Services Program to Meet the Needs of Today” (July 14, 2025)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_PolicyDialogueEvent_20250714_Cancer_01.jpg)
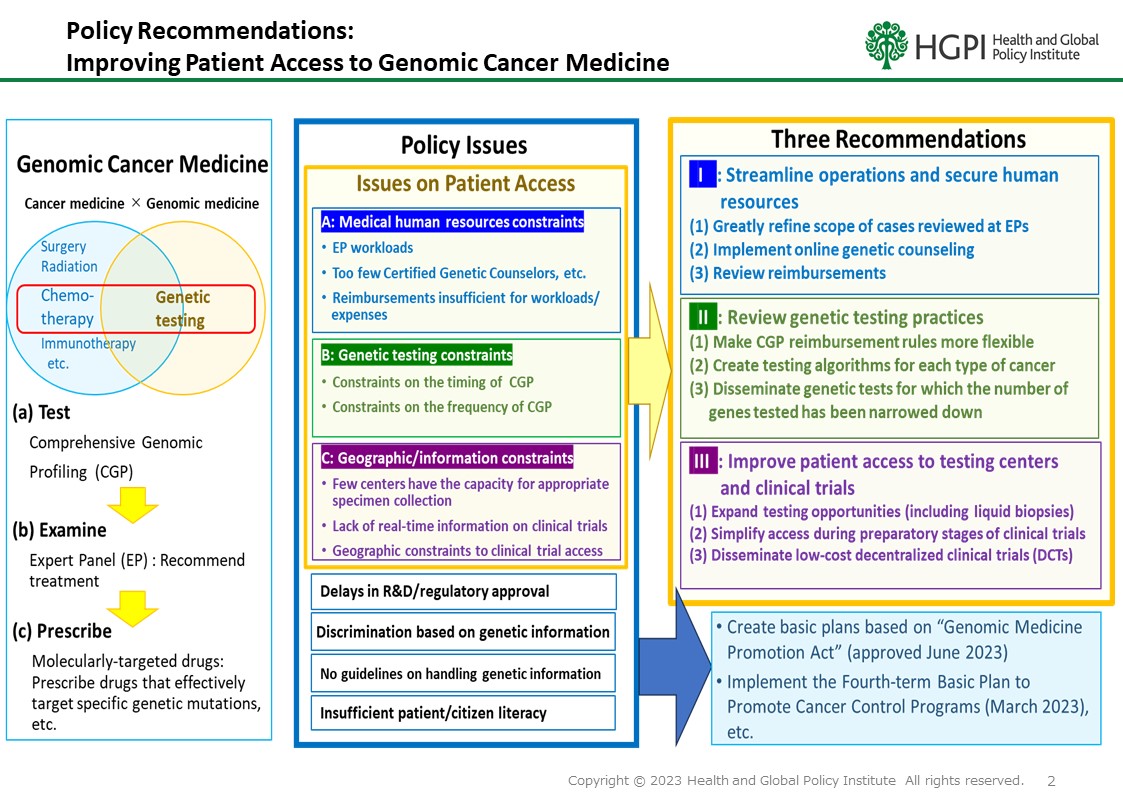
Since the inclusion of cancer gene panel (CGP) testing under public health insurance coverage in 2019, clinical experience and a growing body of research have steadily advanced the field of cancer genomic medicine in Japan. In June 2023, the enactment of the Act on Comprehensive and Planned Promotion of Measures for People to Safely Receive High-Quality and Appropriate Genomic Medicine (commonly known as the “Genomic Medicine Act”) further accelerated nationwide efforts to promote the appropriate use of genomic medicine.
Despite this progress, several challenges remain in ensuring that cancer genomic medicine is widely accessible and appropriately employed for the benefit of all patients. Recognizing these challenges, Health and Global Policy Institute (HGPI) has been engaged in related initiatives since 2021, and in April 2025, we issued a policy recommendation outlining key actions to promote the advancement of cancer genomic medicine.
On June 11, 2025, three leading academic societies, the Japanese Society of Medical Oncology (JSMO), the Japanese Cancer Association (JCA), and the Japanese Society of Clinical Oncology (JSCO), jointly released a “Briefing Report on Solid Cancer Treatment Based on Gene Panel Testing Using Next-Generation Sequencers.” These societies played a critical role in 2019 by providing foundational guidance that contributed to CGP’s initial coverage under health insurance. Their latest report includes recommendations that will serve as a key reference for the upcoming medical fee revision process.
This was an opportune and a pivotal moment to deepen and strengthen cross-sectoral dialogue that will accelerate the translation of cancer genomic medicine into tangible benefits for patients. In light of this, HGPI hosted a roundtable convening stakeholders from industry, government, academia, and civil society to exchange views on the current landscape, ongoing challenges, and future directions of cancer genomic medicine in Japan.
The discussion points from this policy dialogue can be found here.
[Event Overview]
- Date & Time: Monday, July 14, 2025, 18:00-19:30 JST
- Venue: Global Business Hub Tokyo
(3F, Otemachi Financial City Grand Cube, 1-9-2 Otemachi, Chiyoda-ku, Tokyo) - Format: In-person (Invitation-only | Chatham House Rule)
- Organized by: Health and Global Policy Institute (HGPI)
[Program] (Titles Omitted)
18:00-18:10 Opening Remarks, Explanation of Purpose, and Overview of Policy Recommendations
Yui Kohno (Manager, Health and Global Policy Institute)
18:10-18:25 Overview of the Latest Guidance from JSMO, JCA, and JSCO
Manabu Muto (Professor, Department of Medical Oncology, Graduate School of Medicine, Kyoto University)
18:25-19:30 Roundtable Discussion
Designated remarks
Naomi Sakurai (Vice President, Japan Federation of Cancer Patient Groups/ President, CSR Project, General Incorporated Association)
 |
 |
[Participants] (Titles omitted; in Japanese syllabary order)
Hideki Adachi (Executive Manager, External Affairs Dept., Chugai Pharmaceutical Co., Ltd.)
Toyotaka Iguchi (Office Director, Office of New Drug V, Pharmaceuticals and Medical Devices Agency)
Ryota Ichinose (Head of Foundation Medicine Business Dept., Chugai Pharmaceutical Co., Ltd.)
Kenji Iwakabe (Japan Association of Clinical Reagents Industries/ Executive Vice President, LS Business, Sysmex Corporation)
Takayuki Ueki (Senior Manager, EY Strategy and Consulting Co., Ltd.)
Shuichi Kashiwazaki (Japan Association of Clinical Reagents Industries/ Senior Manager, Genetic Science & Clinical Sequencing Division, Clinical Sequencing Department, Thermo Fisher Scientific, Life Technologies Japan Ltd.)
Akira Kamoshida (Section Manager, Product Planning Department, Sompo Himawari Life Insurance Inc.)
Tsutomu Kawaguchi (Sr. Director Medical, JDD&MA Oncology, Eli Lilly Japan K.K.)
Toshiyuki Kihara (Marketing Director, Breast Cancer, Oncology Business Unit, AstraZeneca K.K.)
Naomi Sakurai (Vice President, Japan Federation of Cancer Patient Groups/ President, CSR Project, General Incorporated Association)
Shoji Sanada (Professor and Chairman, Clinical and Translational Research Center, Kobe University Hospital)
Han Xu (Manager, EY Strategy and Consulting Co., Ltd)
Yu Sunakawa (Professor and Chair, Department of Clinical Oncology, St. Marianna University School of Medicine)
Kuniko Sunami (Medical Director, Department of Laboratory Medicine, National Cancer Center Hospital)
Mika Takaki (President & General Manager, Guardant Health Japan Corp.)
Sayuri Takimoto (Associate Director, Government Affairs and Public Policy, AstraZeneca K.K.)
Tomoyuki Takura (Chair and Professor, Division of Health Care Service Management, Nihon University School of Medicine)
Akira Chiba (Assistant Director, Cancer and Disease Control Division; Transplantation Therapy Measures Promotion Office, Intractable/Rare Disease Control Division, Public Health Bureau, Ministry of Health, Labour and Welfare (MHLW))
Kazuko Chono (Fellow, SRL Genomics Division, SRL, Inc.)
Shinya Tsuruta (Director, Cancer and Disease Control Division, Public Health Bureau, Ministry of Health, Labour and Welfare (MHLW))
Kentaro Nakagawa (Head of Medical Affairs, Guardant Health Japan Corp.)
Tessui Nakanishi (Associate Director, Policy Department, Janssen Pharmaceutical K.K.)
Yousuke Nakano (Office of Medicai Innovation, Research and Development Policy Division, Health Policy Bureau, Ministry of Health, Labour and Welfare (MHLW))
Miwa Nishida (Senior Manager, Regulatory Affairs Group, Roche Diagnostics K.K.)
Masami Fujii (Senior Manager, Policy & Public Affairs Japan, Pfizer Japan Inc.)
Kazuyuki Furuta (FoudationOne CDx Lifecycle Leader, Chugai Pharmaceutical Co., Ltd.)
Takeshi Horiike (Senior Deputy Manager, Product Planning Department, Sompo Himawari Life Insurance Inc.)
Takaaki Mizuno (Specially Appointed Assistant Professor, Center for Cancer Genomics, Keio University School of Medicine/ CEO, CLINIAL CO., LTD.)
Manabu Muto (Professor, Department of Medical Oncology, Graduate School of Medicine, Kyoto University)
Takashi Mura (Director, Market Access and Government Affairs, Guardant Health Japan Corp.)
Noriko Morikubo (Japan Regulatory Portfolio Lead, Internal Medicine/Vaccine, Regulatory Strategy Group 1, Regulatory Affairs, Pfizer Japan Inc.)
Daisuke Yasumizu (Director, Head of Policy Department, Janssen Pharmaceutical K.K.)
Yasushi Yatabe (Director, Division of Molecular Pathology, National Cancer Center Hospital)
Top Research & Recommendations Posts
- [Research Report] Perceptions, Knowledge, Actions and Perspectives of Healthcare Organizations in Japan in Relation to Climate Change and Health: A Cross-Sectional Study (November 13, 2025)
- [Research Report] The 2025 Public Opinion Survey on Healthcare in Japan (March 17, 2025)
- [Policy Recommendations] Mental Health Project: Recommendations on Three Issues in the Area of Mental Health (July 4, 2025)
- [Policy Recommendations] Developing a National Health and Climate Strategy for Japan (June 26, 2024)
- [Research Report] The 2023 Public Opinion Survey on Satisfaction in Healthcare in Japan and Healthcare Applications of Generative AI (January 11, 2024)
- [Policy Recommendations] Recommendations on Strategic Investments in Policies for Brain Health to Revitalize Japan: Hopes for the New Administration (December 1, 2025)
- [Policy Recommendations] Reshaping Japan’s Immunization Policy for Life Course Coverage and Vaccine Equity: Challenges and Prospects for an Era of Prevention and Health Promotion (April 25, 2025)
- [Announcement] HGPI Endorses the “Belém Health Action Plan” (November 14, 2025)
- [Announcement] HGPI Joins Global Green and Healthy Hospitals (August 1, 2023)
- [Research Report] Survey of Japanese Nursing Professionals Regarding Climate Change and Health (Final Version) (November 14, 2024)
Featured Posts
-
2025-12-09
[Event Report] Special Seminar “Rising to New Challenges in Health Sciences for Future Society: Novel Developments in the Field of Epilepsy in Japan and Globally” Belgium Pavilion Special Seminar, World Expo 2025 Osaka, Kansai (September 18, 2025)
![[Event Report] Special Seminar “Rising to New Challenges in Health Sciences for Future Society: Novel Developments in the Field of Epilepsy in Japan and Globally” Belgium Pavilion Special Seminar, World Expo 2025 Osaka, Kansai (September 18, 2025)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20250805_mental-health-expo-eyechatch.png)
-
2025-12-11
[Event Report] Core Components of Universal Health Coverage (UHC): Achieving “Healthcare Without Financial Hardship” in Asia-Pacific and Japan (December 5, 2025)
![[Event Report] Core Components of Universal Health Coverage (UHC): Achieving “Healthcare Without Financial Hardship” in Asia-Pacific and Japan (December 5, 2025)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20251210_Core-Components-of-Universal-Health-CoverageUHC-top.jpg)
-
2025-12-12
[Registration Open] Meaningful Involvement Promotion Project Urgent Symposium “The New Takaichi Administration and Central Social Insurance Medical Council Reform – Ensuring Patients’ Voices are Heard” (January 22, 2026)
![[Registration Open] Meaningful Involvement Promotion Project Urgent Symposium “The New Takaichi Administration and Central Social Insurance Medical Council Reform – Ensuring Patients’ Voices are Heard” (January 22, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20251208_urgent-symposium-1.png)
-
2025-12-12
[Registration Open] (Webinar) The 140th HGPI Seminar “Early Detection to Reduce COPD Disease Burden: Connecting Clinical Frontiers with Health Policy” (January 27, 2026)
![[Registration Open] (Webinar) The 140th HGPI Seminar “Early Detection to Reduce COPD Disease Burden: Connecting Clinical Frontiers with Health Policy” (January 27, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/hs140-top.png)
-
2025-12-16
[Discussion Points] Policy Dialogue “Considering Comprehensive Genomic Profiling from the Perspective of Patient Access: Utilizing the Medical Service Fee Reimbursement System and the Mixed Medical Services Program to Meet the Needs of Today” (November 28, 2025)
![[Discussion Points] Policy Dialogue “Considering Comprehensive Genomic Profiling from the Perspective of Patient Access: Utilizing the Medical Service Fee Reimbursement System and the Mixed Medical Services Program to Meet the Needs of Today” (November 28, 2025)](https://hgpi.org/en/wp-content/uploads/sites/2/eyecatch_Policy-Dialogue_Discussion-Points_20251128.jpg)